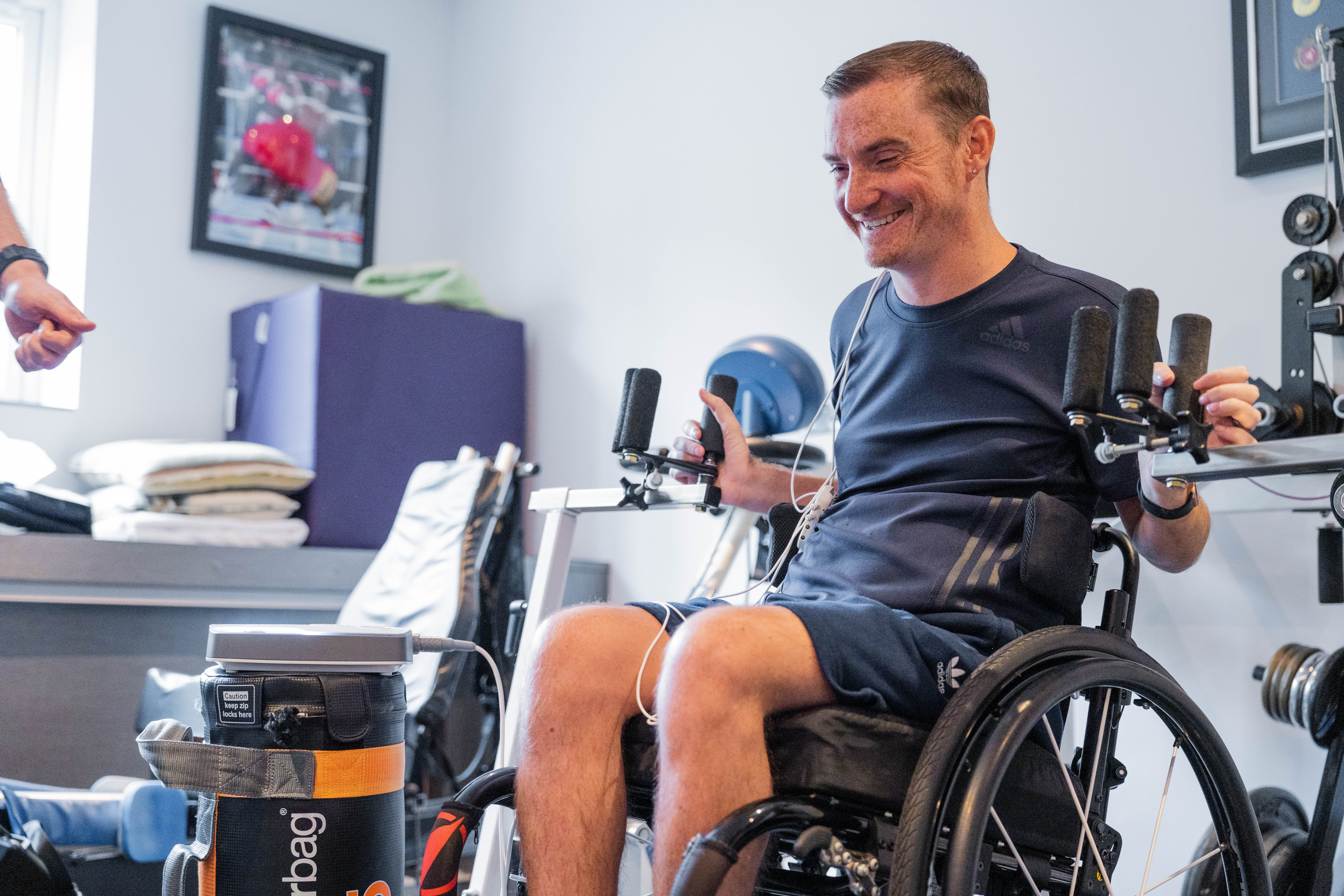
Neurokinex – a provider of activity-based neurorehabilitation – is the first organisation in the UK approved to deliver the ARC-EX System from ONWARD Medical. This positions Neurokinex at the forefront of non-invasive spinal stimulation delivery in a community-based rehabilitation setting.
The arrival of the ARC-EX devices at its centres marks a significant milestone for Neurokinex, which has spent nearly a decade collaborating with academic institutions, clinical groups and industry partners on spinal stimulation research and innovation. The Pathfinder clinical research pilots conducted at Neurokinex centres using ONWARD Medical’s devices saw participants achieve meaningful improvements. Benefitting from this experience, the Neurokinex team of specialist trainers fully appreciates the power of this non-invasive spinal stimulation to bring further gains in function, independence and quality of life for people living with a spinal cord injury.
“For almost 10 years, we have believed that spinal stimulation would play a pivotal role in the future of rehabilitation and long-term wellness for people living with spinal cord injury,” says Harvey Sihota, founder of Neurokinex. “Becoming the first centre in the UK to receive the ONWARD ARC-EX device is an incredibly proud moment for our team and our community. We’re excited to begin integrating this technology into our programmes in the New Year.”
How the ARC-EX system works
The ARC-EX System is the first and only FDA-approved non-invasive technology clinically proven to improve hand strength and sensation for individuals with SCI.
It works by delivering programmed electrical stimulation through the skin to the spinal cord via electrodes placed on the back of the neck. This non-invasive approach requires no surgery, unlike other spinal cord stimulation devices. During therapy sessions, people with SCI use the device while performing functional tasks guided by trained rehabilitation specialists.
The Up-LIFT clinical study published in Nature Medicine showed that 90% of participants improved in upper limb strength or function, with 87% reporting enhanced quality of life. Additional benefits included reduced spasm frequency, improved sleep quality, and better upper body sensation. Regaining hand function is consistently rated as the highest treatment priority for people with spinal cord injuries, as it directly impacts independence in daily activities like eating, dressing and personal care.
“Important moment for spinal cord injury community”
“This is an important moment for the spinal cord injury community that brings technology to life by combining cutting-edge innovation with high-quality, activity-based therapy,” says Harvey. “In the right hands and used alongside existing rehabilitation programmes. I have no doubt ARC-EX stimulation therapy can significantly improve outcomes for people living with a spinal cord injury.”
Neurokinex will offer ARC-EX stimulation therapy alongside its intensive activity-based rehabilitation protocols to clients with chronic, non-progressive spinal cord injuries (C2-C8 inclusive) who are between 18 and 75 years old.
Delivery of ARC-EX stimulation therapy will start from January 2026. People wishing to be considered for this therapy are invited to register using this form: https://form.jotform.com/253512496120350
Image: Neurokinex client benefitting from ONWARD ARC-EX Therapy
Source and image submitted by Neurokinex

